Superior Care Within Standard of Care
Identify and select the most promising treatment options while staying safely within NCCN guidelines.
The SAGE Oncotest™ Difference
The SAGE Oncotest™ is our next generation ex-vivo extreme drug resistance (EDR) assay that provides actionable results from a fresh and viable cancer biopsy within just 7 to 10 days, irrespective of whether the patient’s tumor does or does not have any driver mutations.
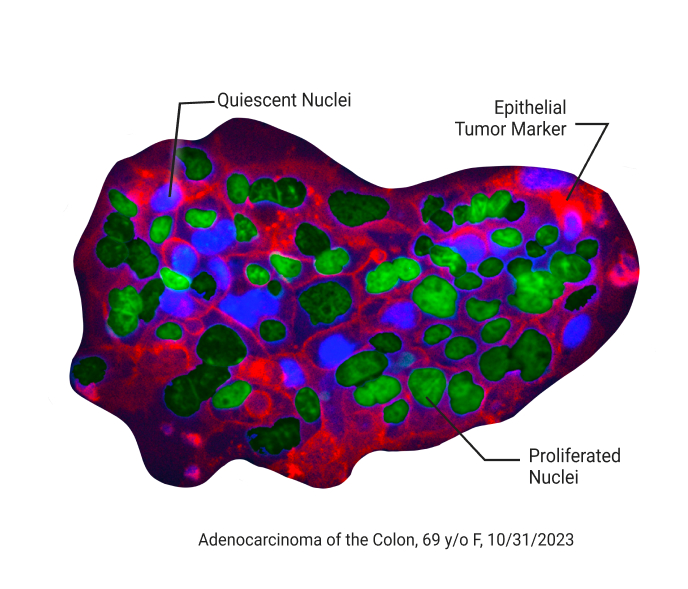
Complement & Overcome Limitations of Genomic Testing
The SAGE Oncotest™ is not based on genomic testing. This is important as only 1 out of 4 patients have actionable driver mutations, whereas the SAGE Oncotest™ can provide guidance in the absence of driver mutations.
It is not a substitute for genomic testing but an essential component to bring precision medicine to the next level to better tailor cancer therapy.
Retaining the Tumor Microenvironment
A surgically excised fresh tissue biopsy is shipped to SAGE overnight. Upon arrival, tissue is processed into hundreds of patient cancer tissue replicates that reflect the heterogeneity of the biopsy. The cancer tissue replicates are then dosed and exposed to the NCCN guideline recommended treatment options for several days. Effectiveness is then reported to the treating physician within days.
1
We create live 3D microtumors of the patient’s sample in a day…
2
that retain the microenvironment and heterogeneity of the sample…
3
testing multiple drugs using our proprietary technologies…
4
to identify the most effective therapy in just one week.
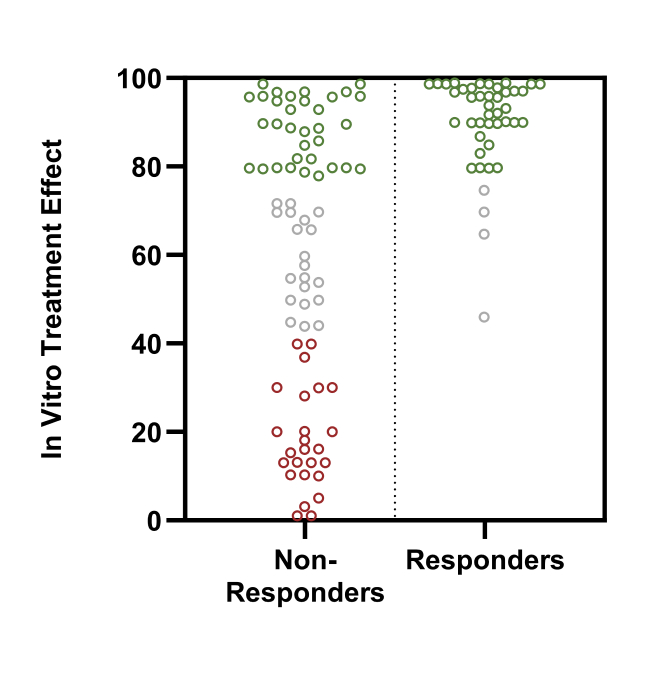
Avoiding Drug Resistance
- Focus on the most promising treatment options by avoiding preexisting drug resistance.
- Our predicate ex-vivo extreme drug resistance (EDR) assay (see figure): Limited response of ex-vivo cancer tissue to supraphysiological doses was associated with clinical cancer resistance in more than 96% of cases.
- Identify multi-drug resistance with more confidence to avoid unnecessary and harmful treatment.
Always Actionable
Identifying Tumor Resistance with High Accuracy
The SAGE Oncotest™ is the next generation of our predicate EDR has demonstrated high accuracy to predict tumor resistance that can be used to avoid unnecessary costs and harm while supporting better patient outcomes.
In our predicate extreme drug resistance assay, the results show less than 60% of treatment effect to a therapy with a higher dose than a patient can tolerate, it’s implausible the patient would benefit from treatment but likely they would be harmed, especially if the patient has already failed the first or second line of treatment.
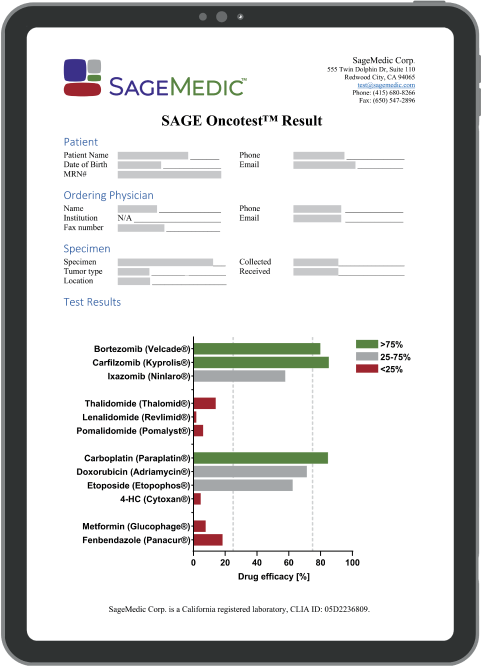
Common Use Cases
A common scenario clinicians face is a number of NCCN recommended regimens with the latitude to choose the most appropriate intervention for each patient.
Beyond Standard of Care
However, clinicians can request testing of additional off-label or repurposed drugs on a case-by-case basis.
Clinicians can use Oncotest™ results to explore options for second- and third-line treatment, expand treatment options earlier for difficult-to-treat solid tumors, avoid cancer resistance, and make timely, evidence-backed decisions.
- Glioblastoma
- Ovarian Cancer
- Gastrointestinal Cancers
- Late-Stage Cancers
- Cancers of Unknown Origin
- Isolating Cancers from Ascites or Pleural Effusions
- Special Requests
Sample Oncotest™ Results
Ovarian Cancer
SAGE Oncotest™ Sample Test
View test results from our testing and analysis of a live ovarian cancer micro-tumor
Rhabdomyosarcoma
SAGE Oncotest™ Sample Test
View sample report of the SAGE Oncotest™ results on sarcoma cancer in the soft tissue.
Multiple Myeloma
SAGE Oncotest™ Sample Test
Test results from a Multiple Myeloma cancer